After the Nobel, what next for Crispr gene-editing therapies?
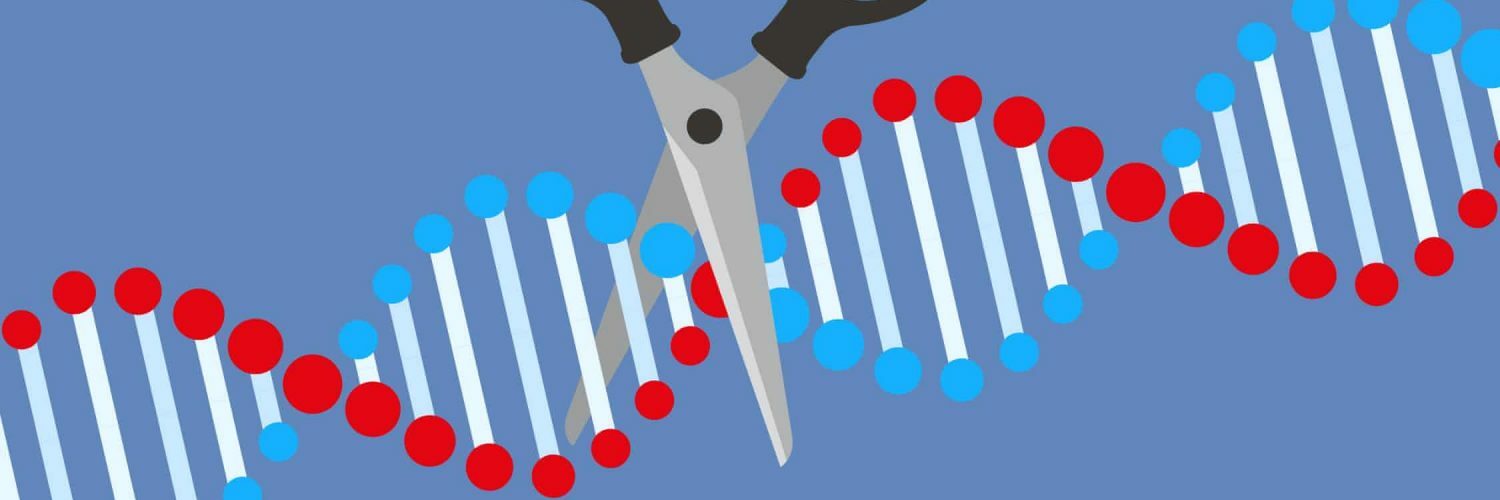
当去年的诺贝尔化学奖被授予生物化学家珍妮弗·杜德纳和微生物学家埃马纽埃尔·沙彭蒂埃,以表彰他们在开发被称为Crispr-Cas9(读作 “crisper”)的基因编辑技术方面的工作时,头条新闻将他们的发现誉为 “分子剪刀”,使我们能够 “改写生命之书”——以及这种能力所引发的所有复杂的伦理问题。但大部分的兴奋点与设计婴儿的愿景无关。Crispr的真正前景是用于治疗由基因突变引起的疾病,从肌肉萎缩症到先天性失明,甚至一些癌症。
When last year’s Nobel prize for chemistry was awarded to biochemist Jennifer Doudna and microbiologist Emmanuelle Charpentier for their work in developing the technique of gene editing known as Crispr-Cas9 (pronounced “crisper”), headlines hailed their discovery as “molecular scissors” that would allow us to “rewrite the book of life” – with all the complicated ethical questions that ability raises. But much of the excitement has nothing to do with visions of designer babies. The real promise of Crispr is for treating diseases caused by genetic mutations, from muscular dystrophy to congenital blindness, and even some cancers.
Crispr疗法已经经历过首次人体试验,研究人员希望它们即将进入临床。加州大学伯克利分校的多德那说:”Crispr研究的进展速度确实令人吃惊。”
Many common diseases, including heart conditions, Alzheimer’s and diabetes, are partly caused by genes: people who inherit the “wrong” variants of certain genes are more vulnerable. For many of these conditions the genetic component is complicated: many genes are involved. Other diseases, such as cystic fibrosis, might be caused by the malfunction of just one or a few genes. In that case, the disease might be cured entirely by gene editing: replacing the faulty genes with the healthy variant.
自从科学家在20世纪70年代首次开始学习如何编辑基因以来,这种 “基因治疗 “方法一直是一个目标。但是它还没有达到不负众望的目的,因为在我们每个细胞的DNA中编辑大约21,000个其他基因是很难的。它需要非常精确的工具来寻找基因,在该点上剪断DNA,然后在其位置上缝合一个新的基因(或一个基因的片段)。
This “gene therapy” approach has been a goal ever since scientists first began learning how to edit genes in the 1970s. But it has never yet lived up to the hype, because editing one gene among about 21,000 others in the DNA of each of our cells is hard. It requires very accurate tools for finding the gene, snipping the DNA at that point, and then stitching in a new gene (or fragment of one) in its place.
Read more at The Guardian